Why test for biomarkers in mNSCLC?
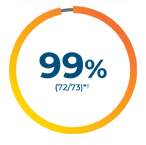
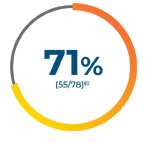
~1 in 2 patients with mNSCLC may have an actionable mutation that can be treated with a targeted therapy7-16*
PD-L1 IS AN IMMUNE CHECKPOINT PROTEIN, NOT AN ACTIONABLE MUTATION17 |
METex14 has a prevalence of ~3%, representing ~4,000-5,000 patients with mNSCLC per year in the United States18,19†
Patients with METex14 in mNSCLC face a poor prognosis20-22
Many of these patients may have bone, liver, and brain metastases, which are associated with poor outcomes
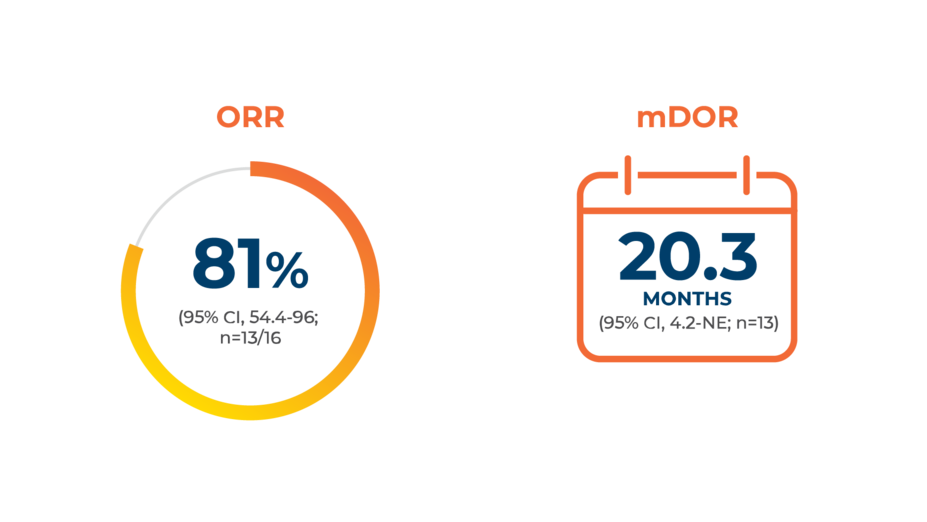
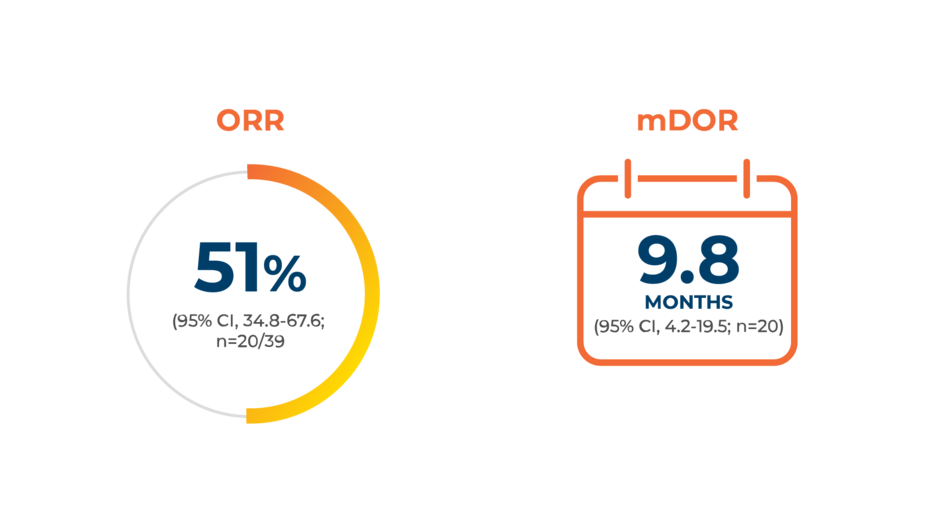
Capmatinib (TABRECTA® tablets) is an NCCN Guidelines®CATEGORY 2A (PREFERRED) FIRST-LINE TREATMENT option for patients with METex14-positive mNSCLC1‡
When to test
To inform up-front treatment planning, test every patient with mNSCLC at diagnosis
According to the NCCN Guidelines for NSCLC, clinicians should obtain biomarker test results in appropriate patients at diagnosis of mNSCLC, prior to administering a first-line therapy, if feasible.1
Accurate detection of mutations leading to METex14 in mNSCLC could facilitate timely intervention23
Identifying METex14 in mNSCLC may unlock the option for first-line targeted therapy with TABRECTA2
How to test
Comprehensive genomic profiling may identify a wide range of actionable mutations with 1 test24,25
Single-gene testing for multiple biomarkers sequentially may:
Result in longer turnaround times and increase the risk of tissue exhaustion, potentially necessitating a rebiopsy26,27
Fail to identify clinically relevant genomic alterations, narrowing the treatment options for patients28
Broad molecular profiling is STRONGLY ADVISED BY NCCN for the identification of actionable mutations in eligible patients with mNSCLC1§
ALK, anaplastic lymphoma kinase; BRAF, v-raf murine sarcoma viral oncogene homolog B1; EGFR, epidermal growth factor receptor; ERBB2, v-erb-b2 avian erythroblastic oncogene homolog 2; HER2, human epidermal growth factor receptor 2; KRAS, Kirsten rat sarcoma; MET, mesenchymal-epithelial transition; METex14, MET exon 14 skipping; mNSCLC, metastatic non-small cell lung cancer; NTRK, neurotrophic tyrosine receptor kinase;
RET, rearranged during transfection; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application.
*Prevalence rates are in accordance with those from The Cancer Genome Atlas (TCGA) Research Network, a joint effort between the National Cancer Institute and the National Human Genome Research Institute. To access the latest TCGA data, please visit: cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga.
†This calculation is based on a 3% prevalence rate and mNSCLC-specific incidence and recurrence data from Kantar Health.
‡See the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for detailed recommendations, including other options.
§The NCCN Guidelines for NSCLC provide recommendations for individual biomarkers that should be tested and recommend testing techniques but do not endorse any specific commercially available biomarker assays or commercial laboratories.