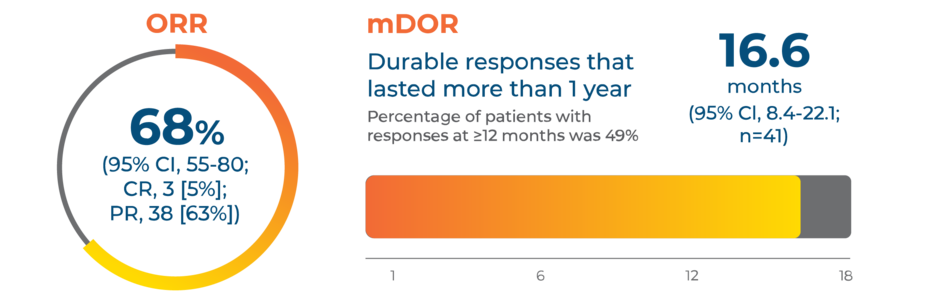
Powerful and sustained responses in treatment-naive patients (n=60)2,3
In the GEOMETRY mono-1 pivotal trial, TABRECTA® (capmatinib) tablets delivered an overall response rate (ORR) of 68% and a median duration of response (mDOR) of over 1 year (16.6 months) in treatment-naive patients (n=60).
CR, complete response; PR, partial response.
Highlights of Important Safety Information
TABRECTA has Warnings and Precautions for interstitial lung disease (ILD/pneumonitis), hepatotoxicity, pancreatic toxicity, hypersensitivity reactions, risk of photosensitivity, and embryo-fetal toxicity.
The most common adverse reactions (≥20%) are edema, nausea, musculoskeletal pain, fatigue, vomiting, dyspnea, cough, and decreased appetite.
Please see additional Important Safety Information below.
Other efficacy outcomes3
The majority of patients taking TABRECTA® (capmatinib) tablets responded within ~7 weeks
98.3% DCR
(95% CI, 91.1-100.0)
Disease control rate (DCR) was an exploratory efficacy outcome that accounted for CR + PR + SD + non-CR/non-PD, which may reflect the natural history of disease in an individual patient rather than the therapeutic effect of the treatment.
Noncomparative analysis of median PFS and OS
Due to the nonrandomized, noncomparative nature of the study, progression-free survival (PFS) and overall survival (OS) results are difficult to interpret. No statistical tests were made for PFS and OS, as there was no comparator arm. PFS and OS results are based on an interim analysis; results are subject to change pending longer trial follow-up.
12.45-month median PFS | 25.49-month median OS |
(95% CI, 8.31-17.97; 37 of 60 [61.7%] event rate) | (95% CI, 15.24-NE; 30 of 60 [50.0%] event rate) |
NE, not estimable; PD, progressive disease; SD, stable disease.
Start strong with TABRECTA® (capmatinib) tablets |